Imidocarb Dipropionate
Chemical Name | :Imidocarb Dipropionate |
Category | :antiprotozoa |
Specification | :In-house |
HS Code | :2933.2900.90 |
- Tel: +86-532-83876123
- Fax: +86-532-83876157
- Email: dennis@qingmeibio.com
- Skype: dennis10221
Structure Formula | :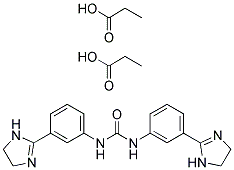 |
CAS Number | :55750-06-6 |
Molecular Formula | :C19H20N6O |
Usage | :Imidocarb Dipropionate, also called double benzene urea, belongs to all diphenyl thiourea derivatives, peptides are three nitrogen to replace the mi a specific, not only the barber to cattle and sheep, insect, pear-shaped insect infection treatment, effect, indeed, and attached to the red cell body of the pig have special effects.This product has a small amount (2 mg/kg bw), easy to use, short course of treatment can be cured (generally 1-2 needle), and low resistance. |
Items | Standard |
|---|---|
Appearance | White or off-white, crystalline powder. |
Identificatio | The retention time for the major peak of sample should correspond to that of reference standard Max.absorption at 240nm It gives the positive reaction of Propionate |
Clarity of Solutio | Clear or not more opalescent than reference suspension I |
Color of Solution | Should not more than reference solution Y4 or YG4 |
pH | 5.5-7.5 |
Water | ≤6.0% |
Related substances | ≤2.0% |
Sulphated Ash | ≤0.1% |
Residual Solvent | ≤0.2% |
Heavy Metals | ≤20ppm |
Assay (On the anhydrous basis) It contains C19H20N6O | ≥ 98.0% |
1, Argentinian National Institute of Drugs
Instituto Nacional de Medicamentos (INAME)
2, Australian Therapeutic Goods Administration (TGA)
3, Belgian Federal Agency for Medicines and Health Products
Agence Fédérale des Médicaments et des Produits de Santé(AFMPS)
Federaal Agentschap voor Geneesmiddelen enGezondheidsproducten (FAGG)
4, Canadian Health Products and Food Branch Inspectorate(HPFBI)
5, Taiwan Food and Drug Administration (TFDA)
7, Czech State Institute for Drug Control
Státní Ústav pro Kontrolu Léčiv (SÚKL)
8, Czech Institute for State Control of Veterinary Biologicals and Medicines (ISCVBM)
9, Danish Health and Medicines Authority (DHMA)
10, Finnish Medicines Agency (FIMEA)
11, French National Agency for Medicines and Health Products Safety
Agence nationale de sécurité du médicament et des produits de santé (ANSM)
12, French Agency for Food, Environmental & Occupational Health Safety
Agence nationale de sécurité sanitaire de l'alimentation, de l'environnement et du travail (ANSES)
13, German Federal Ministry of Health *
Bundesministerium für Gesundheit (BMG)
14, Icelandic Medicines Agency (IMA)
20, Indonesian National Agency for Drug and Food Control (NADFC)
21, Health Products Regulatory Authority (HPRA)
22, Italian Medicines Agency
Agenzia Italiana del Farmaco (AIFA)
23, Japanese Pharmaceuticals and Medical Devices Agency(PMDA)
24, Korea (Republic of) Ministry of Food and Drug Safety (MFDS)
25, Malaysian National Pharmaceutical Control Bureau (NPCB)
26, Dutch Health Care Inspectorate*
Inspectie voor de Gezondheidszorg (IGZ)
27, New Zealand's Medicines and Medical Devices Safety Authority (Medsafe)
28, Norwegian Medicines Agency (NOMA)
29, Polish Main Pharmaceutical Inspectorate (MPI)
30, Romanian National Agency for Medicines and MedicalDevices (NAMMD)
31, South African Medicines Control Council (MCC)
32, Spanish Agency of Medicines and Medical Devices *
Agencia Española de Medicamentos y Productos Sanitarios(AEMPS)
33, Swedish Medical Products Agency (MPA)
34, United Kingdom's Medicines and Healthcare Products Regulatory Agency (MHRA)
35, United Kingdom's Veterinary Medicine Directorate (VMD)
36, U.S. Food and Drug Administration (US FDA)
37, European Directorate for the Quality of Medicines & HealthCare (EDQM)
38, European Medicines Agency(EMA)
39, World Health Organization(WHO)