API / PRODUCT
HOME>Product>Human Health>API
Glimepiride
Chemical Name | :Glimepiride |
Category | : |
Specification | :EP/USP |
HS Code | :29299090.90 |
- Tel: +86-532-83876123
- Fax: +86-532-83876157
- Email: dennis@qingmeibio.com
- Skype: dennis10221
Structure Formula | :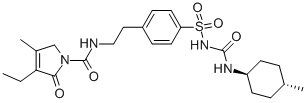 |
CAS Number | :93479-97-1 |
Molecular Formula | :C24H34N4O5S |
Usage | :Glimepiride is the third generation sulfonylurea antidiabetic drug, having the functions of inhibiting synthesis of hepatic glucose, improving insulin-stimulated glucose transport in skeletal muscle and promoting insulin seretion. This product is suitable for type 2 diabetic patient who can’t control blood glucose by diet-treated only, exercise therapy and lose weight. |
Items | Standard |
|---|---|
Appearance | White or almost white powder |
Solubility | Practically insoluble in water; Soluble in dimethylformanide; slightly soluble in methylene chloride, very slightly soluble in methanol. |
Identification IR | Conforms to glimepiride CRS |
Releated substances Impurity B Impurity C Impurity D Specified unidentified RRT 0.67 RRT 0.83 Unsoecified impurity,each sum of impurities other than B |
Not more than 0.4% Not more than 0.1% Not more than 0.2%
Not more than 0.1% Not more than 0.1% Not more than 0.5% |
Impurity A | Not more than 0.4% |
Assay (amhydrous substance) | Not less than 98.0% and not more than 102.0% |
Residual Solvents Acetone | Not more than 2000ppm |
Particle size distribution D100 D90 | Not more than 2000um Not more than 2000um |
We support the below registration services:
1, Argentinian National Institute of Drugs
Instituto Nacional de Medicamentos (INAME)
2, Australian Therapeutic Goods Administration (TGA)
3, Belgian Federal Agency for Medicines and Health Products
Agence Fédérale des Médicaments et des Produits de Santé(AFMPS)
Federaal Agentschap voor Geneesmiddelen enGezondheidsproducten (FAGG)
4, Canadian Health Products and Food Branch Inspectorate(HPFBI)
5, Taiwan Food and Drug Administration (TFDA)
7, Czech State Institute for Drug Control
Státní Ústav pro Kontrolu Léčiv (SÚKL)
8, Czech Institute for State Control of Veterinary Biologicals and Medicines (ISCVBM)
9, Danish Health and Medicines Authority (DHMA)
10, Finnish Medicines Agency (FIMEA)
11, French National Agency for Medicines and Health Products Safety
Agence nationale de sécurité du médicament et des produits de santé (ANSM)
12, French Agency for Food, Environmental & Occupational Health Safety
Agence nationale de sécurité sanitaire de l'alimentation, de l'environnement et du travail (ANSES)
13, German Federal Ministry of Health *
Bundesministerium für Gesundheit (BMG)
14, Icelandic Medicines Agency (IMA)
20, Indonesian National Agency for Drug and Food Control (NADFC)
21, Health Products Regulatory Authority (HPRA)
22, Italian Medicines Agency
Agenzia Italiana del Farmaco (AIFA)
23, Japanese Pharmaceuticals and Medical Devices Agency(PMDA)
24, Korea (Republic of) Ministry of Food and Drug Safety (MFDS)
25, Malaysian National Pharmaceutical Control Bureau (NPCB)
26, Dutch Health Care Inspectorate*
Inspectie voor de Gezondheidszorg (IGZ)
27, New Zealand's Medicines and Medical Devices Safety Authority (Medsafe)
28, Norwegian Medicines Agency (NOMA)
29, Polish Main Pharmaceutical Inspectorate (MPI)
30, Romanian National Agency for Medicines and MedicalDevices (NAMMD)
31, South African Medicines Control Council (MCC)
32, Spanish Agency of Medicines and Medical Devices *
Agencia Española de Medicamentos y Productos Sanitarios(AEMPS)
33, Swedish Medical Products Agency (MPA)
34, United Kingdom's Medicines and Healthcare Products Regulatory Agency (MHRA)
35, United Kingdom's Veterinary Medicine Directorate (VMD)
36, U.S. Food and Drug Administration (US FDA)
37, European Directorate for the Quality of Medicines & HealthCare (EDQM)
38, European Medicines Agency(EMA)
39, World Health Organization(WHO)