Flunixin meglumine
Chemical Name | :Flunixin meglumine |
Category | :anti-inflammatory and analgesic |
Specification | :BP/USP/EP |
HS Code | :3004.9090.82 |
- Tel: +86-532-83876123
- Fax: +86-532-83876157
- Email: dennis@qingmeibio.com
- Skype: dennis10221
Structure Formula | :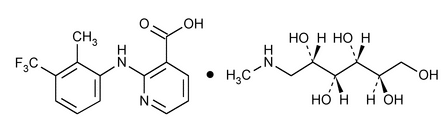 |
CAS Number | :42461-84-7 |
Molecular Formula | :C14H11F3N2O2·C7H17NO5 |
Usage | :Flunixin meglumine is veterinary class anti-inflammatory analgesic.Flunixin meglumine have antipyretic, anti-inflammatory and analgesic effect, alone or with antibiotic combination can significantly improve the clinical symptoms, and can enhance the activity of antibiotics.Veterinary clinical commonly used to relieve the horse's internal cramps, muscle and bone disorders cause pain and anti-inflammatory;Cattle control infection of acute inflammation caused by various diseases, such as laminitis, arthritis, etc., also can be used for sow breast mastitis, metritis, and no syndrome of adjuvant therapy. |
Items | Standard |
|---|---|
Appearance | A white to almost white,crystalline powder |
Solubility | Freely soluble in water and methanol, paractically insoluble in acetone |
Identification 1 | Infrared absorption: 197M; U ltraviolet absorption: 197U |
Identification 2 | Water:FREE SOLUBLE; Ethanol(96%):FREE SOLUBLE; Ethyl acetate:PRACTICALLY INSOLUBLE |
Appearance of solution | Clear and not more intensely coloured than reference solution Y7 |
Melting Point | 137~140°C |
pH | 5%(W/V) water solution; 7.0~9.0 |
Related Substances | 2-chloropyridine-3carboxylic acid ≤0.2% 2-methyl-3-(trifluoromethyl)aniline ≤0.2% ethyl 2-chloropyridine-3-carboxylate ≤0.2% ethyl 2-[[2-methyl-3-(trifluoromethyl) phenyl]amino]pyridine-3-carboxylate ≤0.2% |
Impurities | 1.Single impurity ≤ 0.2% 2.Unknown impurity ≤ 0.2% Total impurities(1~2) ≤ 0.5% |
Specific optical rotation | -9.0º~ -12.0º |
Solvent residue | Acetonitrile ≤ 0.041% Methanol ≤ 0.3% Ethanol ≤ 0.5% Isopropanol ≤ 0.5% Ethyl acetate ≤ 0.5% |
Bacterial endotoxins | ≤ 4.54 EU/mg |
Water | ≤0.5% |
Sulphated Ash | ≤0.1% |
Residual Solvent | ≤0.2% |
colour of solution: | clear, less coloured than reference solution y6, b.p.(Vet) |
1, Argentinian National Institute of Drugs
Instituto Nacional de Medicamentos (INAME)
2, Australian Therapeutic Goods Administration (TGA)
3, Belgian Federal Agency for Medicines and Health Products
Agence Fédérale des Médicaments et des Produits de Santé(AFMPS)
Federaal Agentschap voor Geneesmiddelen enGezondheidsproducten (FAGG)
4, Canadian Health Products and Food Branch Inspectorate(HPFBI)
5, Taiwan Food and Drug Administration (TFDA)
7, Czech State Institute for Drug Control
Státní Ústav pro Kontrolu Léčiv (SÚKL)
8, Czech Institute for State Control of Veterinary Biologicals and Medicines (ISCVBM)
9, Danish Health and Medicines Authority (DHMA)
10, Finnish Medicines Agency (FIMEA)
11, French National Agency for Medicines and Health Products Safety
Agence nationale de sécurité du médicament et des produits de santé (ANSM)
12, French Agency for Food, Environmental & Occupational Health Safety
Agence nationale de sécurité sanitaire de l'alimentation, de l'environnement et du travail (ANSES)
13, German Federal Ministry of Health *
Bundesministerium für Gesundheit (BMG)
14, Icelandic Medicines Agency (IMA)
20, Indonesian National Agency for Drug and Food Control (NADFC)
21, Health Products Regulatory Authority (HPRA)
22, Italian Medicines Agency
Agenzia Italiana del Farmaco (AIFA)
23, Japanese Pharmaceuticals and Medical Devices Agency(PMDA)
24, Korea (Republic of) Ministry of Food and Drug Safety (MFDS)
25, Malaysian National Pharmaceutical Control Bureau (NPCB)
26, Dutch Health Care Inspectorate*
Inspectie voor de Gezondheidszorg (IGZ)
27, New Zealand's Medicines and Medical Devices Safety Authority (Medsafe)
28, Norwegian Medicines Agency (NOMA)
29, Polish Main Pharmaceutical Inspectorate (MPI)
30, Romanian National Agency for Medicines and MedicalDevices (NAMMD)
31, South African Medicines Control Council (MCC)
32, Spanish Agency of Medicines and Medical Devices *
Agencia Española de Medicamentos y Productos Sanitarios(AEMPS)
33, Swedish Medical Products Agency (MPA)
34, United Kingdom's Medicines and Healthcare Products Regulatory Agency (MHRA)
35, United Kingdom's Veterinary Medicine Directorate (VMD)
36, U.S. Food and Drug Administration (US FDA)
37, European Directorate for the Quality of Medicines & HealthCare (EDQM)
38, European Medicines Agency(EMA)
39, World Health Organization(WHO)