Fenbendazole
Chemical Name | : Fenbendazole |
Category | :Anthelmintic |
Specification | :CVP/USP |
HS Code | : |
- Tel: +86-532-83876123
- Fax: +86-532-83876157
- Email: dennis@qingmeibio.com
- Skype: dennis10221
Structure Formula | :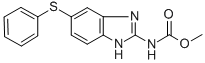 |
CAS Number | :43210-67-9 |
Molecular Formula | :C15H13N3O2S |
Usage | :Gastrointestinal and respiratory nematodes has fine effect of poultry. |
Items | Standard |
|---|---|
Appearance | A white or almost white powder |
Solubility | Practically insoluble in water, sparingly soluble in dimethylformamide, very slightly soluble in methanol. |
Identification | Infrared absorption spectrophotometry |
Impurity A | ≤0.5% |
Impurity B | ≤0.5% |
Any other impurity | ≤0.5% |
Total impurity | ≤1% |
Disregard limit | ≤0.05% |
Heavy metals | ≤20ppm |
Loss on drying | ≤1.0% |
Sulfated ash | ≤0.3% |
Assay | 98.0%~101.0% calculated on the dry basis |
1, Argentinian National Institute of Drugs
Instituto Nacional de Medicamentos (INAME)
2, AustralianTherapeuticGoodsAdministration (TGA)
3, Belgian Federal Agency for Medicines and Health Products
Agence Fédérale des Médicaments et des Produits de Santé(AFMPS)
Federaal Agentschap voor Geneesmiddelen enGezondheidsproducten (FAGG)
4, CanadianHealthProducts andFoodBranchInspectorate(HPFBI)
5, TaiwanFood andDrugAdministration(TFDA)
7, Czech State Institute for Drug Control
Státní Ústav pro Kontrolu Léčiv (SÚKL)
8, CzechInstitute forStateControl ofVeterinaryBiologicalsandMedicines (ISCVBM)
9, DanishHealth andMedicinesAuthority (DHMA)
10, FinnishMedicinesAgency (FIMEA)
11, French National Agency for Medicines and Health Products Safety
Agence nationale de sécurité du médicament et des produits de santé (ANSM)
12, French Agency for Food, Environmental & Occupational Health Safety
Agence nationale de sécurité sanitaire de l'alimentation, de l'environnement et du travail (ANSES)
13, German Federal Ministry of Health *
Bundesministerium für Gesundheit (BMG)
14, IcelandicMedicinesAgency(IMA)
20, IndonesianNationalAgency forDrug andFoodControl (NADFC)
21, HealthProductsRegulatoryAuthority(HPRA)
22, Italian Medicines Agency
Agenzia Italiana del Farmaco (AIFA)
23, JapanesePharmaceuticals andMedicalDevicesAgency(PMDA)
24, Korea (Republic of)Ministry ofFood andDrugSafety(MFDS)
25, MalaysianNationalPharmaceuticalControlBureau(NPCB)
26, Dutch Health Care Inspectorate*
Inspectie voor de Gezondheidszorg (IGZ)
27, New Zealand's Medicines and Medical Devices Safety Authority (Medsafe)
28, NorwegianMedicinesAgency(NOMA)
29, PolishMainPharmaceuticalInspectorate (MPI)
30, RomanianNationalAgency forMedicines andMedicalDevices (NAMMD)
31, South AfricanMedicinesControlCouncil(MCC)
32, Spanish Agency of Medicines and Medical Devices*
Agencia Española de Medicamentos y Productos Sanitarios(AEMPS)
33, SwedishMedicalProductsAgency(MPA)
34, United Kingdom'sMedicines andHealthcareProductsRegulatoryAgency(MHRA)
35, United Kingdom'sVeterinaryMedicineDirectorate (VMD)
36, U.S.Food andDrugAdministration (US FDA)
37, European Directorate for the Quality of Medicines & HealthCare (EDQM)
38, European Medicines Agency(EMA)
39, World Health Organization(WHO)