API / PRODUCT
HOME>Product>Human Health>API
Erythromycin
Chemical Name | :Erythromycin |
Category | :Antibiotic |
Specification | :EP/USP |
HS Code | :29415000.00 |
- Tel: +86-532-83876123
- Fax: +86-532-83876157
- Email: dennis@qingmeibio.com
- Skype: dennis10221
Structure Formula | :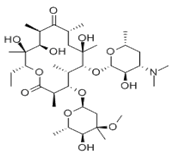 |
CAS Number | :114-07-8 |
Molecular Formula | :C37H67NO13 |
Usage | :In clinical, Progesterone is mainly used in streptococcus pneumoniae caused by tonsillitis, scarlet fever, diphtheria and carrier, gonorrhea, listeria, streptococcus pneumoniae lower respiratory tract infections(The above applies to patients not resistant to penicillin). For legionella pneumonia and mycoplasma pneumonia, this product can be used as the preferred drug application. It can an also be applied to the upper respiratory tract infection caused by influenza bacillus and staphylococcus aureus skin and soft tissue infection, syphilis, and intestinal amebiasis. |
Items | Standard |
|---|---|
Appearance | White or slight yellow powder or colorless of slight yellow crystals |
IR Identification | Infrared spectrum is concordant with that of the reference standard |
Specific optical rotation | -71~-78°(anhydrous substance) |
Related material | Impurity A ≤3.0% Impurity B ≤3.0% Impurity C ≤3.0% Impurity D ≤3.0% Impurity E ≤3.0% Impurity F ≤3.0% Any other impurity ≤0.5% Total impuroties ≤7.0% |
Thiocyanate | ≤0.3% |
Water | ≤0.3% |
Sulfate ash | ≤0.2% |
Assay Erythromycin B Erythromycin C Erythromycin (A+B+C) | ≤5.0% ≤5.0% 93.0%~102.0% |
Residual solvents Methylene chloride | ≤600ppm |
Microbial limit Total Aerobic Microbial Count Total Yeast /Mold Count | ≤cfu10m³/g ≤cfu10² /g |
Items | Standard |
|---|---|
Appearance | White or slight yellow powder or colorless of slight yellow crystals |
IR Identification | Infrared spectrum is concordant with that of the reference standard |
Specific optical rotation | -71~-78°(anhydrous substance) |
Related material | Impurity A ≤3.0% Impurity B ≤3.0% Impurity C ≤3.0% Impurity D ≤3.0% Impurity E ≤3.0% Impurity F ≤3.0% Any other impurity ≤0.5% Total impuroties ≤7.0% |
Thiocyanate | ≤0.3% |
Water | ≤0.3% |
Sulfate ash | ≤0.2% |
Assay Erythromycin B Erythromycin C Erythromycin (A+B+C) | ≤5.0% ≤5.0% 93.0%~102.0% |
Residual solvents Methylene chloride | ≤600ppm |
Microbial limit Total Aerobic Microbial Count Total Yeast /Mold Count | ≤cfu10m³/g ≤cfu10² /g |
We support the below registration services:
1, Argentinian National Institute of Drugs
Instituto Nacional de Medicamentos (INAME)
2, Australian Therapeutic Goods Administration (TGA)
3, Belgian Federal Agency for Medicines and Health Products
Agence Fédérale des Médicaments et des Produits de Santé(AFMPS)
Federaal Agentschap voor Geneesmiddelen enGezondheidsproducten (FAGG)
4, Canadian Health Products and Food Branch Inspectorate(HPFBI)
5, Taiwan Food and Drug Administration (TFDA)
7, Czech State Institute for Drug Control
Státní Ústav pro Kontrolu Léčiv (SÚKL)
8, Czech Institute for State Control of Veterinary Biologicals and Medicines (ISCVBM)
9, Danish Health and Medicines Authority (DHMA)
10, Finnish Medicines Agency (FIMEA)
11, French National Agency for Medicines and Health Products Safety
Agence nationale de sécurité du médicament et des produits de santé (ANSM)
12, French Agency for Food, Environmental & Occupational Health Safety
Agence nationale de sécurité sanitaire de l'alimentation, de l'environnement et du travail (ANSES)
13, German Federal Ministry of Health *
Bundesministerium für Gesundheit (BMG)
14, Icelandic Medicines Agency (IMA)
20, Indonesian National Agency for Drug and Food Control (NADFC)
21, Health Products Regulatory Authority (HPRA)
22, Italian Medicines Agency
Agenzia Italiana del Farmaco (AIFA)
23, Japanese Pharmaceuticals and Medical Devices Agency(PMDA)
24, Korea (Republic of) Ministry of Food and Drug Safety (MFDS)
25, Malaysian National Pharmaceutical Control Bureau (NPCB)
26, Dutch Health Care Inspectorate*
Inspectie voor de Gezondheidszorg (IGZ)
27, New Zealand's Medicines and Medical Devices Safety Authority (Medsafe)
28, Norwegian Medicines Agency (NOMA)
29, Polish Main Pharmaceutical Inspectorate (MPI)
30, Romanian National Agency for Medicines and MedicalDevices (NAMMD)
31, South African Medicines Control Council (MCC)
32, Spanish Agency of Medicines and Medical Devices *
Agencia Española de Medicamentos y Productos Sanitarios(AEMPS)
33, Swedish Medical Products Agency (MPA)
34, United Kingdom's Medicines and Healthcare Products Regulatory Agency (MHRA)
35, United Kingdom's Veterinary Medicine Directorate (VMD)
36, U.S. Food and Drug Administration (US FDA)
37, European Directorate for the Quality of Medicines & HealthCare (EDQM)
38, European Medicines Agency(EMA)
39, World Health Organization(WHO)