Veterinary / PRODUCT
HOME>Product>Animal Health>Veterinary
Enrofloxacin
Chemical Name | :Enrofloxacin |
Category | :Antibiotics |
Specification | :CVP/USP |
HS Code | :29334910.00 |
- Tel: +86-532-83876123
- Fax: +86-532-83876157
- Email: dennis@qingmeibio.com
- Skype: dennis10221
Structure Formula | :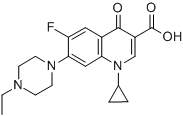 |
Structure Formula | :93106-60-6 |
Molecular Formula | :C19H22FN3O3 |
Usage | :This product is a broad-spectrum sterilization, have special effects on mycoplasma. |
Items | Standard |
|---|---|
Appearance | Pale yellowish or light yellow crystalline powder |
Clarity of solution | The Test solution shows the same clarity as that of water or its opalescence is not more pronounced than that of the Reference suspension |
Color of solution | The Test solution has the appearance of water or is not more intensely colored than the Atandard solution |
Identification | A)IR:The IR spectrum of sample corresponds to that of Enrofloxacin RS B)TLC:The Rf value of the principal spot obtained from the test solution correspond to that obtained from the Standard solution |
Loss on drying | ≤1.0 % |
Residue on ignition | ≤0.1 % |
Chloride | ≤0.04% |
Sulfate | ≤0.04% |
Heavy metals | ≤0.002% |
N-ethyl piperazine | ≤0.1 % |
Fluoroquiolonic acid | ≤0.2% |
Des-fluoro compound | Des-fluoro compound |
Ciprofloxacin | ≤0.3% |
Unspecified impurity | ≤0.1% |
Total impurities(other Fluoroquinolonic acid) | ≤0.5% |
Isoamyl alcohol | ≤0.5% |
DMSO | ≤0.5% |
Ethanol | ≤0.5% |
Assay(on dried basis) | 98.5%~101.5% of C19H22FN3O3 |
1, Argentinian National Institute of Drugs
Instituto Nacional de Medicamentos (INAME)
2, Australian Therapeutic Goods Administration (TGA)
3, Belgian Federal Agency for Medicines and Health Products
Agence Fédérale des Médicaments et des Produits de Santé(AFMPS)
Federaal Agentschap voor Geneesmiddelen enGezondheidsproducten (FAGG)
4, Canadian Health Products and Food Branch Inspectorate(HPFBI)
5, Taiwan Food and Drug Administration (TFDA)
7, Czech State Institute for Drug Control
Státní Ústav pro Kontrolu Léčiv (SÚKL)
8, Czech Institute for State Control of Veterinary Biologicals and Medicines (ISCVBM)
9, Danish Health and Medicines Authority (DHMA)
10, Finnish Medicines Agency (FIMEA)
11, French National Agency for Medicines and Health Products Safety
Agence nationale de sécurité du médicament et des produits de santé (ANSM)
12, French Agency for Food, Environmental & Occupational Health Safety
Agence nationale de sécurité sanitaire de l'alimentation, de l'environnement et du travail (ANSES)
13, German Federal Ministry of Health *
Bundesministerium für Gesundheit (BMG)
14, Icelandic Medicines Agency (IMA)
20, Indonesian National Agency for Drug and Food Control (NADFC)
21, Health Products Regulatory Authority (HPRA)
22, Italian Medicines Agency
Agenzia Italiana del Farmaco (AIFA)
23, Japanese Pharmaceuticals and Medical Devices Agency(PMDA)
24, Korea (Republic of) Ministry of Food and Drug Safety (MFDS)
25, Malaysian National Pharmaceutical Control Bureau (NPCB)
26, Dutch Health Care Inspectorate*
Inspectie voor de Gezondheidszorg (IGZ)
27, New Zealand's Medicines and Medical Devices Safety Authority (Medsafe)
28, Norwegian Medicines Agency (NOMA)
29, Polish Main Pharmaceutical Inspectorate (MPI)
30, Romanian National Agency for Medicines and MedicalDevices (NAMMD)
31, South African Medicines Control Council (MCC)
32, Spanish Agency of Medicines and Medical Devices *
Agencia Española de Medicamentos y Productos Sanitarios(AEMPS)
33, Swedish Medical Products Agency (MPA)
34, United Kingdom's Medicines and Healthcare Products Regulatory Agency (MHRA)
35, United Kingdom's Veterinary Medicine Directorate (VMD)
36, U.S. Food and Drug Administration (US FDA)
37, European Directorate for the Quality of Medicines & HealthCare (EDQM)
38, European Medicines Agency(EMA)
39, World Health Organization(WHO)