Veterinary / PRODUCT
HOME>Product>Animal Health>Veterinary
Ceftiofur Sodium
Chemical Name | :Ceftiofur Sodium |
Category | :Antibiosis |
Specification | :In-house |
HS Code | :3004.2019.11 |
- Tel: +86-532-83876123
- Fax: +86-532-83876157
- Email: dennis@qingmeibio.com
- Skype: dennis10221
Structure Formula | :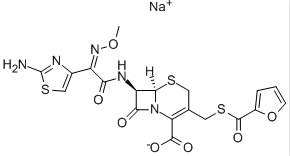 |
CAS Number | :104010-37-9 |
Molecular Formula | :C19H16N5NaO7S3 |
Usage | : Mainly used for fungus poisonous severe infection of e. coli, salmonella and birds.1, viruses, bacteria, systemic or local infection caused by high temperature high fever, cough, gasping, difficulty breathing, skin and red blue and a purple color around the ears and mouth hoof canker, abortion, stillbirth, circle, posterior paresis, don't eat, can't afford to lay down, constipation and diarrhea, etc.2, infectious pleurisy, actinobacillus, kill pasteurella, the more the flow field, salmonella, streptococcus mutans, piglets huangbai vice haemophilus, atrophic rhinitis, chlamydia, etc.3, home storage prenatal postpartum fever, endometritis, mastitis, breast milk syndrome, less laminitis pasteurella rotting hoof inflammation, cattle, sheep, heat transport, pneumonia, nasal contraction, etc. |
Items | Standard |
|---|---|
Appearance | White to grayish yellow powder,odor free and humidifying |
Identification | Inthe chromatogram of the assay,the retention time of the principal peak in the chromatogram obtained with the test solution corresponds to that of the test solution corresponds to that of the principal peak in the chromatogram obtained with reference solution. |
Color of solution | No more intensely colored than reference solution Yellow 9 or Orange-Yellow 9 |
Clarity of solution | No more pronounced than that of reference suspension I |
ph | 5.5-7.5 |
Related substances | Biggest individual impurity ≤ 0.5% Total ≤ 3.0% |
Water | ≤3.0% |
Sulphated Ash | ≤2.0% |
Residual Solvent | Acetone ≤2.0% |
Bacteria Endotoxins | ≤0.2EU/mg |
Assay (On the anhydrous basis)It contains C19H16N5NaO7S3 | ≥98.0% |
1, Argentinian National Institute of Drugs
Instituto Nacional de Medicamentos (INAME)
2, Australian Therapeutic Goods Administration (TGA)
3, Belgian Federal Agency for Medicines and Health Products
Agence Fédérale des Médicaments et des Produits de Santé(AFMPS)
Federaal Agentschap voor Geneesmiddelen enGezondheidsproducten (FAGG)
4, Canadian Health Products and Food Branch Inspectorate(HPFBI)
5, Taiwan Food and Drug Administration (TFDA)
7, Czech State Institute for Drug Control
Státní Ústav pro Kontrolu Léčiv (SÚKL)
8, Czech Institute for State Control of Veterinary Biologicals and Medicines (ISCVBM)
9, Danish Health and Medicines Authority (DHMA)
10, Finnish Medicines Agency (FIMEA)
11, French National Agency for Medicines and Health Products Safety
Agence nationale de sécurité du médicament et des produits de santé (ANSM)
12, French Agency for Food, Environmental & Occupational Health Safety
Agence nationale de sécurité sanitaire de l'alimentation, de l'environnement et du travail (ANSES)
13, German Federal Ministry of Health *
Bundesministerium für Gesundheit (BMG)
14, Icelandic Medicines Agency (IMA)
20, Indonesian National Agency for Drug and Food Control (NADFC)
21, Health Products Regulatory Authority (HPRA)
22, Italian Medicines Agency
Agenzia Italiana del Farmaco (AIFA)
23, Japanese Pharmaceuticals and Medical Devices Agency(PMDA)
24, Korea (Republic of) Ministry of Food and Drug Safety (MFDS)
25, Malaysian National Pharmaceutical Control Bureau (NPCB)
26, Dutch Health Care Inspectorate*
Inspectie voor de Gezondheidszorg (IGZ)
27, New Zealand's Medicines and Medical Devices Safety Authority (Medsafe)
28, Norwegian Medicines Agency (NOMA)
29, Polish Main Pharmaceutical Inspectorate (MPI)
30, Romanian National Agency for Medicines and MedicalDevices (NAMMD)
31, South African Medicines Control Council (MCC)
32, Spanish Agency of Medicines and Medical Devices *
Agencia Española de Medicamentos y Productos Sanitarios(AEMPS)
33, Swedish Medical Products Agency (MPA)
34, United Kingdom's Medicines and Healthcare Products Regulatory Agency (MHRA)
35, United Kingdom's Veterinary Medicine Directorate (VMD)
36, U.S. Food and Drug Administration (US FDA)
37, European Directorate for the Quality of Medicines & HealthCare (EDQM)
38, European Medicines Agency(EMA)
39, World Health Organization(WHO)