API / PRODUCT
HOME>Product>Human Health>API
Acarbose
| Chemical Name | : Acarbose |
| Category | : Hypoglycemic |
| Specification | : EP |
| HS Code | : 2932.9990.99 |
- Tel: +86-532-83876123
- Fax: +86-532-83876157
- Email: dennis@qingmeibio.com
- Skype: dennis10221
| Structure Formula | : 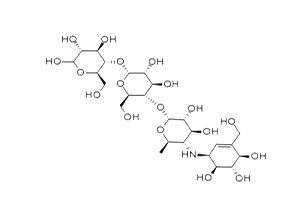 |
| CAS Number | : 56180-94-0 |
| Molecular Formula | : C25H43NO18 |
| Usage | : Acarbose is a novel oral hypoglycemic agents. In the intestine of competitive inhibition of glycoside hydrolase. Reduce the polysaccharide and sucrose into glucose, the sugar uptake corresponding to slow down, so it can be with the postprandial blood glucose lowering effect. Generally used alone, or with other oral hypoglycemic agents or insulin therapy. With the food and beverage, treatment of insulin dependent and non dependent diabetes mellitus. Acarbose for carbohydrates in the small intestine and is absorbed slow decomposition, prolong the retention time, the intestinal bacteria glycolysis and gas production increased, so it can cause abdominal distension, abdominal pain and diarrhea |
| Items | Standard |
|---|---|
| Appearance | White or yellow, amorphous powder, hygroscopic. Very solution in water, soluble in methanol, practically insoluble in methylene chloride |
| Identification | As per EP7.0 |
| Accordant with IR spectrum obtained | |
| with reference standard | |
| Absorbance | Max.0.15 at 425nm for solution S |
| Assay(on anhydrousbasis) | 95.0%-102.0% |
| Water | ≤4.0% |
| Specific optical rotation | +168°~+183° |
| Sulfated ash | ≤0.2% |
| PH | 5.5~7.5 |
| Heavy metals | ≤20ppm |
| Impurity A | ≤0.6% |
| Impurity B | ≤0.5% |
| Impurity C | ≤1.5% |
| Impurity D | ≤1.0% |
| Impurity E | ≤0.2% |
| Impurity F | ≤0.3% |
| Impurity G | ≤0.3% |
| Impurity H | ≤0.2% |
| Any other impurity | Each impurities ≤0.2% |
| Total impurities | ≤3.0% |
| Bulk density | 0.50-0.65g/ml |
| Tapped density | 0.70-0.90g/ml |
| Total plate count | ≤1000vfu/g |
| Mould | ≤100vfu/g |
We support the below registration services:
1, Argentinian National Institute of Drugs
Instituto Nacional de Medicamentos (INAME)
2, Australian Therapeutic Goods Administration (TGA)
3, Belgian Federal Agency for Medicines and Health Products
Agence Fédérale des Médicaments et des Produits de Santé(AFMPS)
Federaal Agentschap voor Geneesmiddelen enGezondheidsproducten (FAGG)
4, Canadian Health Products and Food Branch Inspectorate(HPFBI)
5, Taiwan Food and Drug Administration (TFDA)
7, Czech State Institute for Drug Control
Státní Ústav pro Kontrolu Léčiv (SÚKL)
8, Czech Institute for State Control of Veterinary Biologicals and Medicines (ISCVBM)
9, Danish Health and Medicines Authority (DHMA)
10, Finnish Medicines Agency (FIMEA)
11, French National Agency for Medicines and Health Products Safety
Agence nationale de sécurité du médicament et des produits de santé (ANSM)
12, French Agency for Food, Environmental & Occupational Health Safety
Agence nationale de sécurité sanitaire de l'alimentation, de l'environnement et du travail (ANSES)
13, German Federal Ministry of Health *
Bundesministerium für Gesundheit (BMG)
14, Icelandic Medicines Agency (IMA)
20, Indonesian National Agency for Drug and Food Control (NADFC)
21, Health Products Regulatory Authority (HPRA)
22, Italian Medicines Agency
Agenzia Italiana del Farmaco (AIFA)
23, Japanese Pharmaceuticals and Medical Devices Agency(PMDA)
24, Korea (Republic of) Ministry of Food and Drug Safety (MFDS)
25, Malaysian National Pharmaceutical Control Bureau (NPCB)
26, Dutch Health Care Inspectorate*
Inspectie voor de Gezondheidszorg (IGZ)
27, New Zealand's Medicines and Medical Devices Safety Authority (Medsafe)
28, Norwegian Medicines Agency (NOMA)
29, Polish Main Pharmaceutical Inspectorate (MPI)
30, Romanian National Agency for Medicines and MedicalDevices (NAMMD)
31, South African Medicines Control Council (MCC)
32, Spanish Agency of Medicines and Medical Devices *
Agencia Española de Medicamentos y Productos Sanitarios(AEMPS)
33, Swedish Medical Products Agency (MPA)
34, United Kingdom's Medicines and Healthcare Products Regulatory Agency (MHRA)
35, United Kingdom's Veterinary Medicine Directorate (VMD)
36, U.S. Food and Drug Administration (US FDA)
37, European Directorate for the Quality of Medicines & HealthCare (EDQM)
38, European Medicines Agency(EMA)
39, World Health Organization(WHO)